
| Mercury | |
| Electrons per shell | 2,8,18,32,18,2 |
| Electron Configuration | [Xe] 4f14 5d10 6s2  |
| Ground state | 1S0 |
| Atomic Volume | 14.82 cm3 mol-1 |
| Electronegativity | 2.00 |
| Magnetic ordering | Dimagnetic |
| Mass magnetic susceptibility | -2.1 x 10-9 |
| Molar magnetic susceptibility | -4.21 x 10-10 |
| Speed of sound | 1451.4 m s-1 |
 Thermal Properties Thermal Properties |
|
| Enthalpy of Atomization | 61.5 kJ mol-1 @25°C |
| Enthalpy of Fusion | 2.33 kJ mol-1 |
| Enthalpy of Vaporisation | 59.1 kJ mol-1 |
| Heat Capacity | 27.983 J mol-1 K-1 |
| Thermal Conductivity | 8.30 W m-1 K-1 |
| Thermal Expansion | 60.4 μm m-1 K-1 |
 Electrical Properties Electrical Properties |
|
| Electrical resistivity | 9.6 x 10-7 Ω m |
| Electrical conductivity | 0.0104 106/cm Ω |
 Chemical Properties Chemical Properties |
|
| Electrochemical Equivalent | 3.742 g Ah-1 |
| Electron Work Function | 4.49 eV |
| Valence Electron Potential (-eV) | 28.2 |
| Ionization Potential | |
| 10.437 | |
| 18.759 | |
| 34.202 | |
| Incompatibilities | Acetylene (C2H2), ammonia (NH3), chlorine dioxide (ClO2), azides, calcium (amalgam formation), sodium carbide (NaC), lithium, rubidium, copper. |
 Flammability Flammability |
|
 Optical Properties Optical Properties |
|
| Refractive Index (gas) | 1.000933 |
| Reflectivity | % |
 Energy Levels Energy Levels |
|
| 70.819 KeV | |
| 68.895 KeV | |
| 80.253 KeV | |
| 9.9888 KeV | |
| 9.8976 KeV | |
| 11.8226 KeV | |
| 11.9241 KeV | |
| 11.9928 KeV | |
| 11.5626 KeV | |
| 13.8301 KeV | |
| 14.1588 KeV | |
| 10.647 KeV | |
| 8.7215 KeV | |
 Atomic Radii Atomic Radii |
|
| 150 pm | |
| 171 pm | |
| 149 pm | |
| 155 pm | |
| No data. | |
| 151 pm | |
 Oxidation States Oxidation States |
|
| Hg+2 | |
| Hg+1 | |
 Ionisation Energies (kJ mol-1) Ionisation Energies (kJ mol-1)  |
|
| 1007 | |
| 1809 | |
| 3300 | |
| 4400 | |
| 5900 | |
| 7400 | |
| 9100 | |
| 11600 | |
| 13400 | |
| 15300 | |
 Vapour Pressure Vapour Pressure |
||||||
| P (Pa) | 1 | 10 | 100 | 1K | 10K | 100K |
| T (K) | 315 | 350 | 393 | 449 | 523 | 629 |
 Crystal Structure Crystal Structure |
|
| Rhombohedral | |
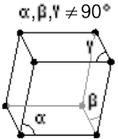 |
a = 300.5 pm |
| b = 300.5 pm | |
| c = 300.5 pm | |
| α = 70.520° | |
| β = 70.520° | |
| γ = 70.520° | |